X-RaY
ANALYSES OF FAMILY 8 CHITOSANASE FROM BACILLus
SP. K17
Wataru Adachi, Shinji Shimizu,
Tomoko Sunami, Tesuya Fukazawa, Mamie Suzuki,
Rie Yatsunami, Satoshi Nakamura
and Akio Takénaka
Graduate School of Bioscience
and Biotechnology, Tokyo Institute of Technology, Nagatsuta, Midori-ku,
Yokohama 226-8501, Japan (wadachi@bio.titech.ac.jp)
Glycosylases
are classified into 86 families according to the characteristics of their amino
acid sequences. Chitosanase from Bacillus sp. K-17 (C-K17) belongs to the family 8 and differs from other
chitosanases in cleavage site specificity. The family 8 also includes cellulase, xylanase and
lichenase. Sequence comparison was
difficult to distinguish the difference of their catalytic site. The active site of C-K17 must, however,
have some difference in their three-dimensional structures. To understand the structural evolution
of the enzymes in the family 8, X-ray analyses of C-K17 have been performed at
different pH.
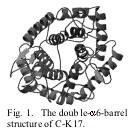 The two structures of the
active (pH 6.4) and inactive (pH3.7) forms are almost the same except the Glu74
residue that have two conformations at pH3.7, suggesting its inactiveness. The architecture of the enzyme is
composed of a double-a6-barrel
structure, the protruded loops from the b-sheets making a large cleft for binding the substrate.
The two structures of the
active (pH 6.4) and inactive (pH3.7) forms are almost the same except the Glu74
residue that have two conformations at pH3.7, suggesting its inactiveness. The architecture of the enzyme is
composed of a double-a6-barrel
structure, the protruded loops from the b-sheets making a large cleft for binding the substrate.
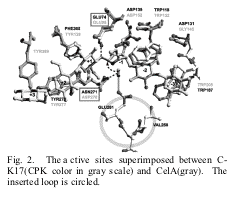 A structural comparison
between cellulase from Clostridium thermocellum
(CelA) and C-K17 shows that their overall structures are similar, but different
in the cleft regions with insertions of additional b-sheets and loops. The
Asp residue, which acts as a proton acceptor in CelA, is changed to Asn271 in
C-K17. Instead, the inserted
Glu261 residue is located close the catalytic site as a proton acceptor.
A structural comparison
between cellulase from Clostridium thermocellum
(CelA) and C-K17 shows that their overall structures are similar, but different
in the cleft regions with insertions of additional b-sheets and loops. The
Asp residue, which acts as a proton acceptor in CelA, is changed to Asn271 in
C-K17. Instead, the inserted
Glu261 residue is located close the catalytic site as a proton acceptor.
Sequence
alignment based on the above structural feature shows that proteins in the
family 8 are classified into the three subfamilies (A, B and C) according to
the proton acceptor, chitosanases and lichenases being in the subfamily A,
whereas cellulases and xylanases in the subfamily B. It is thus concluded that the common ancestor of proteins in
the family 8 is diverged into three subfamilies by inserting a loop to change
the specificity of catalytic reactions.