Three novel polymeric network of
copper(II) constructed with succinato ligand and the influences of weak
interactions on their crystal packing
Tian-Huey Lu,a G.
Mostafaa and N. Ray Chaudhurib
aDepartment. of Physics,
National Tsing Hua University, Taiwan, R.O.C.; bDepartment. of Inorganic Chemistry, IACS, W.B., India
(thlu@phys.nthu.edu.tw)
The crystal engineering of metal organic coordination network is now
a growing field. The key objective of the advanced crystal engineering is the
control on manipulation of weak interactions in order to tune the properties of
the bulk material to design and prepare new functional materials, such as
molecular-based magnets etc. Thus, to synthesize new solid phases with
predictable stoichiometry and architecture for specific applications, one has
to understand the underlying factors such as hydrogen bonding, p-p, C-HÉp interactions etc. that determine
crystal packing. Till date, an accurate prediction of the overall crystal
structure currently is almost impossible but it may be achieved in the near
future. For this reason a large database needed to obtain the crystal packing
rules for a specific classes of functional material.
With this background, the carboxylate group is one of the most
widely used bridging ligands for designing polynuclear complexes with
interesting magentic properties. The versatility of a carboxylate group can be
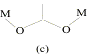
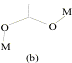
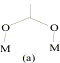
illustrated by the variety of bridging conformations, the most important being
(a)syn-syn, (b)syn-anti and (c)anti-anti.
Of the carboxylato groups, major studies have been done on oxalato
bridged metal complexes with a few examples of terepthalate, fumarate, maleate,
malonate, adipate etc but the reports on succinate and glutarate as
superexchange pathways are scanty in literature. These carboxylates showed wide
varieties of the superexchange phenomenon and are closely related to the
bridging conformations adopted by the carboxylate group in polynuclear systems.
To design a carboxylato bridged pre-assigned functional material, hence to
achieve a particular bridging conformation, a systematic study and more
examples of carboxylato bridged complexes are needed.
Here, we wish to report the relevance of the succinato ligand for
the construction of novel network structure of Cu(II). The weak interactions,
observed here to form the solid-state phases of this class of magnetic
material, will be discussed. In complexes 1 and 2, a weak ferromagnetic path is
observed but the global behavior is antiferromagnetic.
Crystal
Data:
(1) [Cu2(m-OH2)2(m-succinate)(bipy)(NO3)2]n, Triclinic, P![]() , a=7.0641(6), b=9.8410(8), c=10.4885(9) , α=72.079(2)0, b=73.176(2)0, γ=74.6290(10)0
, a=7.0641(6), b=9.8410(8), c=10.4885(9) , α=72.079(2)0, b=73.176(2)0, γ=74.6290(10)0
(2) [Cu2(m-OH2)2(m-succinate)( phen)(NO3)2]n, Triclinic, P![]() , a=6.9386(8), b=10.3463(14), c=10.8572(17) , α=69.734(11)0,
b=81.354(11)0, γ=75.687(10)0
, a=6.9386(8), b=10.3463(14), c=10.8572(17) , α=69.734(11)0,
b=81.354(11)0, γ=75.687(10)0
(3) [{Cu8(m-succinate)4(phen)12}(BF4)4]n, Triclinic, P![]() , a=12.5769(10), b=
19.3034(15), c=19.3348(16) , α=60.187(2) 0, b=86.864(2) 0, γ=80.538(2) 0
, a=12.5769(10), b=
19.3034(15), c=19.3348(16) , α=60.187(2) 0, b=86.864(2) 0, γ=80.538(2) 0