MECHANISMS OF
SELF-ASSEMBLY AND SWITCHING OF THE BACTERIAL FLAGELLUM
Keiichi Namba
Graduate School of Frontier Biosciences, Osaka
University Protonic NanoMachine Project, ERATO, JST, &
Dynamic NanoMachine Project, ICORP, JST, 3-4 Hikaridai, Seika, Kyoto 619-0237
Japan (keiichi@fbs.osaka-u.ac.jp)
The bacterial flagellum is
made of a rotary motor and a long helical filament by means of which bacteria
swim. The size of the bacterial cell body is about 1 mm by 2 mm, but the
flagellum grows to about 15 mm long. The flagellar motor at its base rotates at
around 300 Hz and drives the rapid rotation of each flagellum to propel the
cell movements in viscous environments. The diameter of the flagellar motor is
30 to 40 nm, ant it consists of many proteins including membrane spanning
proteins: a rotor ring, made of about 25 copies of FliF/FliG complex; about
eight stator units, made of MotA/MotB complex; other parts such as the rotation
switch regulator, bushing, and drive shaft, all made of different proteins. The
long helical filament, which is a tubular structure with a diameter of about 20
nm, is made of 20,000 to 30,000 copies of a single protein flagellin, and yet
the filament can form left-handed or right-handed helical forms and switch
between these two in response to the twisting force produced by quick reversal
of the motor rotation. This allows bacteria to alternate their swimming pattern
between running and tumbling, which is essential for their tactic behavior. The
flagellum also has a short, highly curved segment that connects the motor and
the helical propeller, and this segment is called hook. Its bending flexibility
makes it function as a universal joint, while the filament is relatively more
rigid to work as a propeller. There is a very short segment called the
hook-filament junction, which is made of HAP1 and HAP3. This junction is thought
to play a mechanical buffer to connect the two mechanically distinct
structures. The flagellum is constructed through various self-assembly
processes, in which all the axial structures growing towards the cell exterior
are constructed by the flagellar component proteins translocated from the
cytoplasm to the distal end of the growing structure, where three cap complexes
help efficient self-assembly of these proteins in different stages.
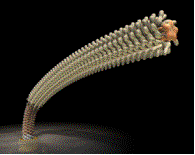
 We have been trying to
visualize the structure of the flagellum in atomic detail to understand how it
self-assembles and works. We solved crystal structures of core fragments of the
flagellar axial component proteins by X-ray crystallography. X-ray fiber
diffraction gave high-resolution structural information. Electron
cryomicroscopy also visualized the structures of the filament, cap and
cap-filament complex. All these structures present interesting implications for
the function of each molecule, demonstrating the importance of dual nature of
protein molecules, flexibility and precision.
We have been trying to
visualize the structure of the flagellum in atomic detail to understand how it
self-assembles and works. We solved crystal structures of core fragments of the
flagellar axial component proteins by X-ray crystallography. X-ray fiber
diffraction gave high-resolution structural information. Electron
cryomicroscopy also visualized the structures of the filament, cap and
cap-filament complex. All these structures present interesting implications for
the function of each molecule, demonstrating the importance of dual nature of
protein molecules, flexibility and precision.