UNEXPECTED PIPERAZINE
DERIVATIVE LIGANDS FROM A MIXTURE OF CuCl2.2H2O, Na2CO3,
AND TRIETHYLENETETRAMINE TETRAHYDRO- CHLORIDE
Norihiro Tamura,a Masato
Sakai,b Katsuya Kudoh,b Goro Hiharab and Hiroshi
Miyamaeb
aSchool of
Dentistry, Meikai University, Saitama 350-0283, Japan; bDepartment of
Chemistry, Josai University, Saitama 350-0295, Japan (miya@josai.ac.jp)
We have already reported that PbCl2
can remove contaminated 2,2Õ,2Ó- triaminotriethylamine (tren) from commercially
available triethylenetetramine (trien) [1]. There are another stories of
impurities in trien.
One of us mixed CuCl2.2H2O
with trien in water, the mixture gave a complex cation as shown in Fig. 1(1) with [CuCl3]2-
anion. There is a ligand, 1-(6,3-diaza-
hexyl)piperazine, which act as a tridentate with the terminal N of the
piperidine ring being protonated.
The starting Cu compound contains Cu(II) ion, but in the anion the Cu
atom should be oxidation state of +1.
The result indicates that the reaction must contain a redox step which
might has relation to form a new ligand.
The other trial to produce compound 1 gave us two
nuclear Cu complex as shown in Fig. 2(2), when we had used
excess of Na2CO3. The ligand is 1,4-bis(2-aminoethyl)piperazine
which act as bidentate to each Cu and make bridging. There is no indication for redox process, but the excess of
CO32- ion seems to compensate the positive charge of the
Cu(II) ions.
It is uncertain whether the organics come
originally from impurities or from reaction products of the complex
formation. However it is one of
the good procedures to get the ligands, which are not commercially available.
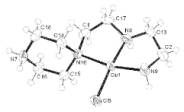
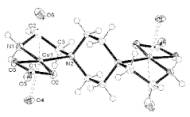
Fig. 1. The structure of 1. Fig.
2. The structure of 2.
References
1
Miyamae, H., Yoshinari, K., Hihara, G. and Nagata, M. (1988)
Acta Cryst. C44, 1528-1530.