Charge density studies of polymorphic anti-ulcer
agents. the applicability of the electrostatic potential in drug design
Jacob
Overgaard, Mark P. Waller and David E. Hibbs
School
of Chemistry, University of Sydney, NSW 2006, Australia
(jacobo@chem.usyd.edu.au)
The electrostatic potential (EP) has been
extensively employed in the prediction of a variety of condensed phase
macroscopic properties from theoretical calculations, and a quantitative
approach has recently been suggested based on a range of features of the EP on
the molecular surface [1]. However, this method has so far been restricted to
the gas-phase, thus excluding the effect of intermolecular interactions.
Nonetheless, the EP is of paramount importance in the understanding of
drug-receptor interactions. Thus, an experimental determination of the EP
including the effects of intermolecular interactions is potentially of great use
in rational drug design.
In the present work we will outline the
results of a theoretical and experimental charge density (CD) study of both
known polymorphs (A and B) of the histamine H2-receptor antagonist,
famotidine (see Figure) [2]. The CD is determined from a combination of X-ray
and neutron diffraction data collected at 100 K, using the Hansen-Coppens
multipole model [3]. We will focus on a comparison of the experimental and
theoretical CDs and describe the similarities in the CDs of the two polymorphic
forms of famotidine. In particular, we will discuss the observed differences in
the experimental EPs of the two polymorphs, respectively, in relation to their
individual abilities to act as anti-ulcer agents. This work represents the
preliminary steps towards a more general description of a number of drug types
using combined theoretical and experimental charge density studies.
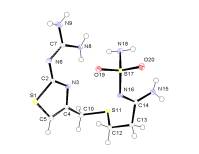
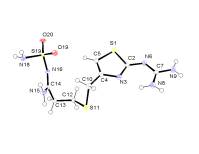
Famotidine B
Famotidine
A
References:
1
Politzer, P.,
Murray, J. S. (2002) Theor. Chem. Acc. 108, 134-142.
2
Ferenczy, G.
G., Parkanyi, L. J., Angyan, G., Kalman, A., Hegedus, B. (2000) J. Mol.
Struct. (Theochem) 503, 73-79.
3
Hansen, N.
K., Coppens, P. (1979) Acta Crystallogr. Sect. A 39, 909-921.